SAFE
Plasma-Functionalized SurfAce for Bio-Medical Environment
The innovation brought about by the SAFE project lies in the development and combination of wet and dry plasma surface treatment technologies.

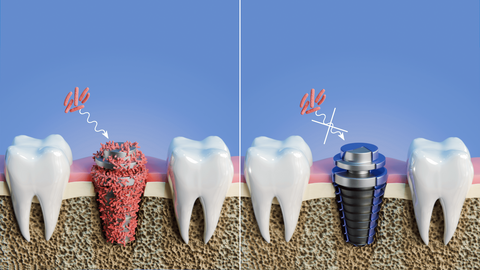
Biomaterials are used to manufacture medical implants, particularly those used in orthopedic surgery (screws, plates...), vascular surgery (stents...) and dental implantology (screws, abutments...). They can be used to replace a lost or diseased biological structure, or to restore function or form. In this way, they help to improve the quality of life of an ageing population. Titanium (Ti)-based alloy implants are widely used in the dental field, where they face real clinical problems linked to slow biological response to their surface, slow osseointegration and bacterial colonization leading to loosening of the implant and consequent loss of viability. The performance requirements for implants are: good mechanical properties, controlled resistance to corrosion in biological environments, antibacterial properties and adequate biological response. Surface modifications are therefore necessary to optimize their functional properties.
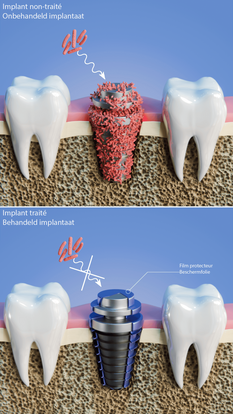
In this context, the innovation brought by the SAFE project lies in the development and combination of wet and dry plasma surface treatment technologies, to micro- and nanostructure the titanium surface in order to meet the demanding specifications of clinicians and hospitals, but also to innovate in the development of biomaterials. The proposed combination of plasma technologies has not yet been considered in other projects, and will enable a TRL level of between 3 and 7 to be achieved for titanium dental implants and an endoscopic device. To achieve this objective, the project will draw on the complementary expertise of the cross-border teams. These include experts in wet and dry processing technologies (UMONS and UGent), technology and performance upscaling (Materia Nova and CRITT), as well as morphological and mechanical analysis of materials and surfaces (ULille, UPHF, Materia Nova and UMONS).
The SAFE project will also provide the medical and industrial communities with a characterization technology platform that will help them in the development of new biomaterials and in the dissemination of research results thanks to the partnership initiated with associated operators (CHULille, CH EpiCura, CHU Helora, UZGent, BioWin).
| Department(s) | Partner(s) | Overall amount |
|---|---|---|
|
2,810 k€
|
||
| Main support | Rayout | Date(s) |
| INTERREG | European |
2024 - 2028
|


